UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
|
|
|
|
|
|
|
(State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(I.R.S. Employer Identification No.) |
(Address of principal executive offices, including zip code)
(
(Registrant’s telephone number, including area code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
|
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
|
|
|
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
|
|
|
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
|
|
|
|
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
|
|
|
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
|
|
Title of each class |
|
Trade Symbol(s) |
|
Name of each exchange on which registered |
|
|
|
|
|
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
|
Item 7.01. |
Regulation FD Disclosure. |
Included as Exhibit 99.1 to this Current Report on Form 8-K is an updated corporate presentation for Galecto, Inc. (the “Registrant”), dated January 2022, which is incorporated herein by reference. We intend to utilize this presentation and its contents in various meetings with securities analysts, investors and others commencing on January 5, 2022.
The information in this Current Report on Form 8-K, including Exhibit 99.1, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
|
Item 9.01. |
Financial Statements and Exhibits. |
(d) Exhibits
|
Exhibit Number |
|
Description |
|
|
|
|
|
99.1 |
|
|
|
|
|
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
|
Galecto, Inc. |
||
|
|
|
|
|
|||
|
Date: January 5, 2022 |
|
|
|
By: |
|
/s/ Hans T. Schambye |
|
|
|
|
|
|
|
Hans T. Schambye, M.D., Ph.D. |
|
|
|
|
|
|
|
President and Chief Executive Officer |

First-in-class small-molecule anti-fibrotic and anti-cancer agents January 2022 Exhibit 99.1

Forward-looking statements This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding Galecto, Inc.’s (the “Company”) strategy, future operations, future financial position, projected costs, prospects, plans, and objectives, are forward-looking statements. Such forward-looking statements include statements about the GALACTIC-1 trial, plans for continuing to enroll patients, working with investigators and regulatory authorities, the timing of completing enrollment and the initial unblinded data readout, GB0139’s potential (including the effectiveness of the 3 mg dose), plans for clinical development and potential to market, as well as Galecto’s product candidates and pipeline. The words ‘‘anticipate,’’ ‘‘believe,’’ ‘‘continue,’’ ‘‘could,’’ ‘‘estimate,’’ ‘‘expect,’’ ‘‘intend,’’ ‘‘may,’’ ‘‘plan,’’ ‘‘potential,’’ ‘‘predict,’’ ‘‘project,’’ ‘‘target,’’ ‘‘should,’’ ‘‘would,’’ and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. For such statements, we claim the protection of the Private Securities Litigation Reform Act of 1995. The Company may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements. Factors that could cause actual results to differ materially from such statements include, without limitation: our ability to continue to enroll patients and complete the GALACTIC-1 trial with fewer dosage groups; the risk that FDA or other regulatory agencies impose a clinical hold on the GALACTIC-1 trial; that drug development is expensive, time consuming, uncertain and susceptible to change, interruption, delay or termination; the duration and severity of the ongoing coronavirus disease (COVID-19) pandemic, including but not limited to the impact on our clinical and other operations, the operations of our suppliers, others and the capital markets, which in each case remains uncertain; that the timing and outcome of research, development and regulatory review and feedback is uncertain; our need to raise additional capital to advance all of our programs; the amount of our future losses is uncertain and could cause our stock price to fluctuate or decline; top-line data may not accurately reflect the complete results of a particle study or trial; results of clinical trials and other studies are subject to different interpretation and may not be predictive of future results; new data or results may be unexpected or unfavorable; our drug candidates may not advance in development or be approved for marketing; clinical trial and other studies may not proceed at the time or in the manner expected or at all; enrolling patients in our ongoing and intended clinical trials is competitive and challenging; clinical and nonclinical data is voluminous and detailed, and regulatory agencies may interpret or weigh the importance of data differently and reach different conclusions than we or others, request additional information, have additional recommendations or change their guidance or requirements; data and information related to our program may not meet regulatory requirements or otherwise be sufficient for further development at all or on our projected timeline; and other risks related to developing, seeking regulatory approval of and commercializing drugs, including regulatory, manufacturing, supply and marketing issues and drug availability. Additional factors that could cause results to differ materially from those stated or implied by our forward-looking statements are disclosed in our Securities and Exchange Commission (SEC) filings, including our most recent Annual Report on Form 10-K, filed with the SEC on March 29, 2021, under the headings “Risk Factors.” In addition, the forward-looking statements included in this presentation represent the Company’s views as of the date of this presentation. The Company anticipates that subsequent events and developments will cause its views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so.

Investment highlights Small-molecule inhibitors targeting Galectin-3 & lysyl oxidase-like 2 (LOXL2) First company with a potent and selective galectin inhibitor tested in humans Strong and broad IP coverage including composition of matter into the late 2030’s without patent term extension Clinical-stage biotechnology company committed to the development of novel small-molecule therapeutics for the treatment of fibrosis & cancer Phase 2 trials in IPF, myelofibrosis & liver cirrhosis ongoing Lead asset GB0139: a potentially first-in-class with FDA and EMA orphan drug designation (ODD) Phase 2 study in NSCLC to be initiated in 1H 2022 – collaboration with Roche Deep pipeline with meaningful upcoming catalysts Cash balance on September 30, 2021 of ~$128M, funded into 2H 2024 3

Deep pipeline of assets targeting fibrosis and cancer GALACTIC-1 (Inhaled Galectin-3 inhibitor) MYLOX-1 (Oral LOXL2 inhibitor) GALLANT-1 (Oral Galectin-3 inhibitor) GULLIVER-2 (Oral Galectin-3 inhibitor) 4

Experienced management team Hans Schambye CEO 5 Anders Pedersen Bertil Lindmark Pernille Aasholm Garrett Winslow Jon Freve Becky Smith Stephanie Oestreich COO CMO Head of People GC CFO VP, Operational Strategy CBO

Overlapping biology in fibrosis and cancer through Galectin-3 and LOXL2 Galectin-3 and LOXL2 are master regulators fueling key pathological pathways in fibrosis and cancer ECM proliferation Angiogenesis M2 Macrophages Collagen crosslinking Chemo/CPI resistence Cancer stemness Metastatic potential Cell proliferation Ebrahim et al (2014); Ann Transl Med;2(9):88; Farhad et al (2018); Oncoimmunology;7(6):e1434467; Vuong et al (2019); Cancer Res;79;1480; Chang et al (2017), Oncotarget; 8(16):26066; MacKinnon et al (2012); and Am J Respir Crit Care Med;185:537. Slack et al., (2021) Int J Biochem Cell Biol;130:105881 Fibrosis Cancer 6

7 GB0139: Inhaled Galectin-3 Inhibitor for IPF

Approximately 100K patients in US IPF is a progressive, irreversible, ultimately fatal lung disease characterized by decline in lung function (as measured by forced vital capacity (FVC)) Lung tissue scars and becomes non-functional Median survival of 2-5 years Death caused by respiratory failure Unknown cause IPF is a large orphan indication with suboptimal solutions Disease Overview Limited Treatment Options Only two approved drugs slow disease progression: pirfenidone and nintedanib Neither has been associated with improvements in overall survival Both have significant side-effects that limit compliance and usage Due to side effects, less than 50% of patients on treatment Despite dose-limiting side effects, sales of pirfenidone and nintedanib exceeded $3.7B and $2.8B in 2020 and 2019, respectively 8

Galectin-3 impacts key elements in the fibrosis cascade - inhibited by our Galectin-3 inhibitors 9

GB0139 GB0139 delivered to the periphery of the lungs at high concentrations GB0139 targets macrophages – the cells driving the fibrosis mechanism Unique and pluripotent MoA GB0139 inhibits fibrosis by targeting macrophages, fibroblasts, and epithelial cells GB0139 reduced macrophage galectin-3 levels in lungs of IPF patients Dose-response effects on several fibrosis plasma biomarkers No other therapy in development has demonstrated similar consistent effects Inhaled therapy via generic inhaler delivers therapy directly to target tissue with low systemic exposure Other clinical development candidates given intravenously, subcutaneously and orally Superior Delivery Pluripotent MoA Confirmed Target Engagement Indications of Efficacy GB0139: Inhalable, once-daily treatment for IPF Potential for accelerated approval 10
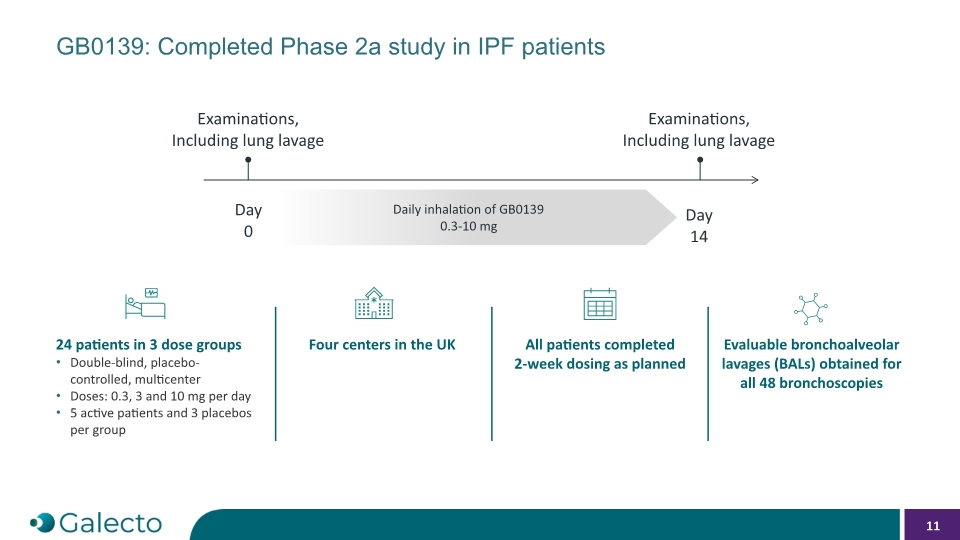
GB0139: Completed Phase 2a study in IPF patients Day 0 Day 14 Daily inhalation of GB0139 0.3-10 mg Examinations, Including lung lavage Examinations, Including lung lavage 24 patients in 3 dose groups Double-blind, placebo-controlled, multicenter Doses: 0.3, 3 and 10 mg per day 5 active patients and 3 placebos per group Four centers in the UK All patients completed 2-week dosing as planned Evaluable bronchoalveolar lavages (BALs) obtained for all 48 bronchoscopies 11

GB0139: Phase 2a result - Bioavailability & target engagement in IPF patients Exceptionally Consistent PK Data in IPF Patients GB0139 Reaches the Alveolar Macrophages Deep within Lung Extracellular Gal-3 Levels Alveolar Macrophages GB0139 Concentration in Alveolar Macrophages Induces Profound Reduction of Gal-3 Levels on Alveolar Macs 12

Biomarkers associated with IPF disease severity and progression had biggest impact Biomarker effects cited by EMA as clinically relevant in IPF patients and basis for orphan drug designation (ODD) BAL fluid and plasma correlation indicates GB0139 directly impacts lung function GB0139: Phase 2a study showed consistent, dose-dependent effects on highly relevant fibrosis biomarkers PDGF-BB PAI-1 YKL-40 PDGF-AA MCP-1 13

GALACTIC-1: Ongoing Phase 2b study in IPF patients Randomized, placebo controlled 52-week study 100+ centers Primary outcome measure: Annualized rate of Forced Vital Capacity (FVC) decline over 52 weeks Study is powered to see an effect in patients not on pirfenidone or nintedanib Key secondary outcomes: Safety, Diffusing Capacity for Carbon Monoxide (DLCO), 6-minute walk test, Quality of Life Randomized 2:1 GB0139 to Placebo 14 GB0139 3mg Placebo Final efficacy analysis Rate of FVC decline Randomization w0 w52 w4 w8 w12 w26 -w12 w32 w40

GB0139: Inhaled Galectin-3 inhibitor summary Ground-breaking novel treatment for IPF, an orphan disease with poorly tolerated treatments Inhaled, delivered directly to the site of active lung destruction Reaches the target cell in the lung at high concentrations Promising biomarker trends observed in Phase 2a study - validated by EMA as clinically relevant in IPF patients and basis for ODD Causes a dose-related reduction in cell surface galectin-3 deep within patient lungs 15

GB1211: Oral Galectin-3 Inhibitor for Cancer 16

Galecto’s oral Galectin-3 inhibitors Galectin-3 is a very attractive target for a series of currently poorly treated indications, including many cancer types Lead compound, GB1211, reduced fibrosis and cancer growth in several animal models Well-tolerated in IND-enabling studies Galecto plans to initially develop GB1211 for both NSCLC and liver cirrhosis Phase 1 SAD/MAD in healthy volunteers successfully completed Well-tolerated, no SAEs and highly suitable PK profile 17 Galecto, as a pioneer in the galectin field, has developed a series of orally active, specific and potent inhibitors of galectin-3

Galectin-3 expression predicts response to pembrolizumab in NSCLC 34 patients with PD-L1 +ve NSCLC stage IV received pembrolizumab (200 mg IV @ 3 wks) High galectin-3 expression in patients with NSCLC strongly correlated with tumor resistance to pembrolizumab A clinical response was seen in tumors with a negative, low or intermediate galectin-3 expression Galectin-3 expression in NSCLC biopsies 18

Increased levels of tumor Galectin-3 significantly drives the hallmarks of cancer 19 M2 Macrophages Cell proliferation Apoptosis Metastatic potential Cancer stemness Angiogenesis ECM proliferation Cytotoxic T cells Increased Galectin-3 Adapted from: Ebrahim et al (2014); Ann Transl Med;2(9):88 Farhad et al (2018); Oncoimmunology;7(6):e1434467 Vuong et al (2019); Cancer Res;79;1480 Gal-3 Chemo/CPI resistance

Increased growth, progression, angiogenesis and metastasis Head and Neck Cancer Cell proliferation, anti-apoptosis, immune escape Gastric Cancer Enhances gastric cell motility and mediates metastasis Cervical Cancer Mediates resistance to chemotherapy Lung Cancer Tumor growth, metastasis, immune suppression, predicts response to CPI therapy Melanoma Renal Cell Cancer Anti-apoptosis, resistance to chemotherapy Bladder Cancer Increases malignant potential Tumor progression and tumor evasion Pancreatic Cancer Ovarian Cancer Mediates resistance to chemotherapy Tumor progression, vascular invasion and metastasis Hepatocellular Carcinoma Galectin-3 is a key driver in multiple oncology indications Galectin-3 modulates tumor growth and immunosuppression in the tumor microenvironment Ebrahim et al, Ann Transl Med 2014;2(9):88; Dubé-Delarosbil et al, Cell Mol Life Sci; (2018);75:1215; Kindt et al, Int. J Mol Sci (2017); 18, 2745; and Song et al, Br J Cancer (2020);123:1521 20
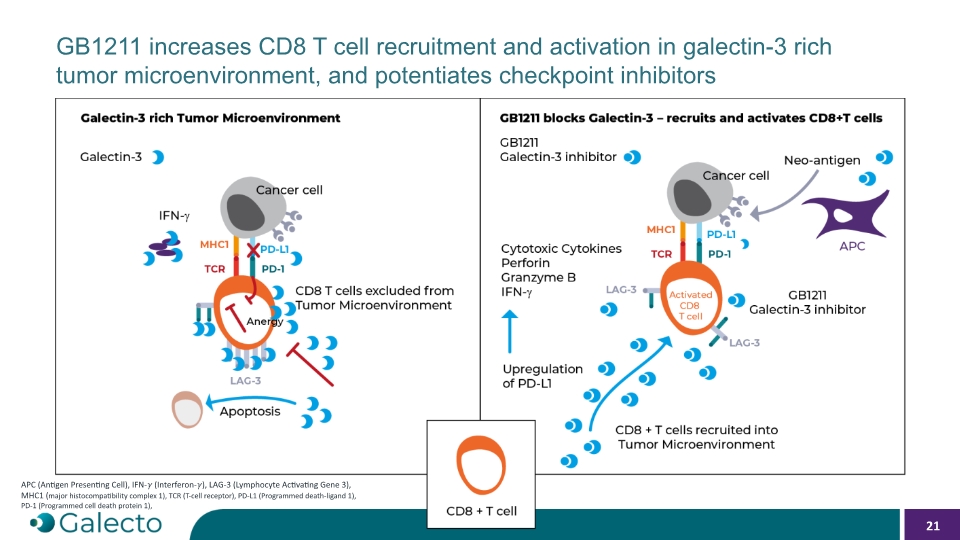
GB1211 increases CD8 T cell recruitment and activation in galectin-3 rich tumor microenvironment, and potentiates checkpoint inhibitors 21 APC (Antigen Presenting Cell), IFN-�� (Interferon-��), LAG-3 (Lymphocyte Activating Gene 3), MHC1 (major histocompatibility complex 1), TCR (T-cell receptor), PD-L1 (Programmed death-ligand 1), PD-1 (Programmed cell death protein 1),

Galecto has chosen NSCLC as first development target NSCLC represents a significant unmet medical need with a strong rationale for anti-Galectin-3 therapy High unmet need Lung cancer is 2nd most common cancer and leading cause of cancer death More than 130.000 death/year in US 1.59 million death/year globally NSCLC has a poor prognosis – 5-year survival <25% Metastatic NSCLC: 5-year survival rate < 7% Billion-dollar market opportunity Galectin-3 is a promising target that Predicts overall poor survival Predicts response to CPI therapy CPI therapy for treatment of NSCLC is well established However, 40-60% of patients don't respond to therapy Gal-3 inhibitors show: Anti-tumor effects T cell activation – LAG3 blockade Macrophage polarizations Increased apoptosis ASCO: Cancer.net (01-2021) Ebrahim et al (2014); Ann Transl Med;2(9):88 Kuou et al (2015); Cancer Immunol Res;3: 412 Ou et al (2021); Ther Adv Med Oncol;13: 1 Capalbo et al. (2019); Int. J. Mol. Sci.;20 Vuong et al (2019); Cancer Res;79: 1480 22

Patients receiving GB1211 400 mg + atezolizumab (25 patients) Patients receiving placebo + atezolizumab (25 patients) Screening Part C Part B Part A GALLANT-1 (Phase 2a) study design Primary efficacy measure is tumor shrinkage Randomisation Primary Outcome -2 weeks screening 12 weeks blinded treatment Continuation of blinded treatment until last patient has received his/her 12-week treatment Long term safety follow-up Unblinding after the last patient has received 12 weeks treatment Continued treatment with atezolizumab (and GB1211) until loss of clinical benefit 23 Dose escalation Dose escalation

Galecto oncology opportunities 24 Inhibition of galectin-3 may have anti-tumor potential in combination with CPIs, chemo- and radiotherapy - And as monotherapy Galectin-3 plays central role for the hallmarks of cancer and is linked to poor survival for many solid tumors Galectin-3 is a negative regulator of immune cell functions and drives low CPI response rate GB1211 is a specific oral galectin-3 inhibitor ready for Phase 2 Anti-tumor effects in preclinical models Well-tolerated and no observed adverse safety or drug interaction signals Galecto collaborating with Roche on upcoming NSCLC first line trial with GB1211 Randomized, placebo-controlled trial in combination with Tecentriq® Planned initiation 1H 2022 with expected topline data mid 2023 GB1211 marks Galecto’s first entry into the solid tumor space

GB2064: LOXL2 Inhibitor for Myelofibrosis and Other Oncology and Fibrotic Diseases 25

GB2064 A small-molecule inhibiting LOXL2, an enzyme that catabolizes the formation of lysine cross-linking in fibrillar collagens Potentially disease modifying Opportunity in multiple fibrotic indications Myelofibrosis Orphan indication: 16,000 - 18,500 patients in US Current therapies (JAK inhibitors) are not disease modifying Large market - Incyte’s Jakafi® achieved sales of $1.9B and $1.7B in 2020 and 2019, respectively GB2064: Oral LOXL2 inhibitor in myelofibrosis Overview and Treatment Opportunity LOX Family Gene Expression in Myelofibrosis Stromal Cells LOXL2 paralogue most upregulated in active myelofibrosis 26

GB2064 is a small-molecule inhibitor of the LOXL2 enzyme catalytic site, not an antibody approach GB2064 therefore has the potential to avoid the in vivo target low tissue penetration and low target engagement seen with Gilead’s simtuzumab Simtuzumab maximal enzyme inhibition of 40% at the clinically unattainable 1µM level GB2064’s superior efficacy to simtuzumab has been observed in cell-based assays and preclinical models GB2064: Demonstrated in vitro inhibition of LOXL2 GB2064 GB2064 fully inhibits LOXL2 activity using a variety of substrates Max enzyme inhibition by Simtuzumab Max enzyme inhibition by GB2064 27

MYLOX-1: GB2064 in myelofibrosis First patient dosed in Q3 2021 Study to be led by Professor Srdan Verstovsek, MD Anderson Single arm, open label study allowing real-time read of safety and activity Planned for 16 evaluable patients initially for 9 months of treatment Patients who are ineligible for or who do not tolerate JAK-inhibitors Planned endpoints for readout include: Blood formation Bone marrow general histology and fibrosis PK to show drug levels in target tissue Imaging for spleen and liver volume GB 2064 1000mg 2x daily for 9 months 28

GB2064: Phase 2a in myelofibrosis summary Ample evidence for central role of LOXL2 in fibrosis and cancer GB2064 potently inhibits LOXL2 and shows antifibrotic activity in numerous models Current Phase 2a trial could generate both target engagement and efficacy data as repeated biopsies are already standard practice Opportunity for both orphan drug designation and fast track designation following data in this indication Phase 2a trial started in Q3 2021 Phase 1 SAD/MAD study completed Chronic toxicology studies completed Robust efficacy in lung, liver and kidney fibrosis models Expected topline data in 2H 2022 29

GB1211: Oral Galectin-3 Inhibitor for Liver Cirrhosis 30

GB1211: Oral Galectin-3 inhibitor for advanced liver cirrhosis Disease Overview Cirrhosis prevalence: ~2 million patients in US, ~3 million in EU1 Severe, progressive liver fibrosis ultimately leads to liver failure Caused primarily by NASH, alcoholic liver disease and viral hepatitis Median survival of ~2 years for decompensated cirrhosis2 Limited treatment options: Resolving etiology may improve decompensation e.g., alcohol abstinence, HCV/HBV antivirals Liver transplantation 31 1 https://www.thelancet.com/journals/langas/article/PIIS2468-1253(19)30349-8/fulltext Sepanlou, et al Lancet Gastroenterol Hepatol 2020; 5: 245–66 2 https://www.hepatitis.va.gov/cirrhosis/background/stages.asp

GB1211: Oral Galectin-3 inhibitor for advanced liver cirrhosis Galectin-3 in Advanced Cirrhosis Evidence links galectin-3 to cirrhosis progression: Galectin-3 is elevated in alcoholic and non-alcoholic cirrhosis and in toxic hepatitis Galectin-3 is prognostic biomarker of hepatocellular carcinoma, a known complication of liver cirrhosis Preclinical data suggest galectin-3 inhibition may address cirrhosis: Inhibition of galectin-3 reduces development of fibrosis Galectin-3 is required for TGF-ß mediated myofibroblast activation and matrix production in liver fibrosis Galectin-3 inhibition reduces YKL-40, a biomarker that is elevated with progressive liver fibrosis 32 1 https://www.thelancet.com/journals/langas/article/PIIS2468-1253(19)30349-8/fulltext Sepanlou, et al Lancet Gastroenterol Hepatol 2020; 5: 245–66 2 https://www.hepatitis.va.gov/cirrhosis/background/stages.asp

Gulliver-2 study design GB1211 100mg (n=15) Moderate hepatic impairment (n=6) Severe hepatic impairment (n=6) Part 1 (Completed Q4 2021) Successfully completed open-label, single dose study Part 2 (Initiated Q4 2021) Randomized, double blind, placebo-controlled 12-week repeat dose (2x daily) Placebo (n=15) Part 3 Open-label, single dose study Matched healthy controls (n=6) Matched healthy controls (n=6) Screen GB1211 Single dose 100mg D1 D2 D3 D4 D10/11 Follow up 6-7 days post last PK sample SRC D1 D7 D21 D42 D84 D63 Randomized Follow up W1 W3 W6 W9 W12 Screen GB1211 Single dose 100mg D1 D2 D3 D4 D10/11 Follow up 6-7 days post last PK sample 33 Study in Child-Pugh B/C patients designed to illustrate both safety and drug activity on fibrosis and liver function SRC: Safety Review Committee Endpoints - changes in PK, biomarkers for collagen, FibroScan (liver & spleen), liver functional capacity test

GB1211: Oral Galectin-3 inhibitor for liver cirrhosis GB1211 has completed clinical Phase 1 testing SAD/MAD in healthy volunteers successfully completed Hepatic impairment study completed in Q4 2021 Current status & next steps IND opened in Q1 2020 Phase 2a study initiated in Q4 2021 Expected topline data in 2H 2022 Extensive preclinical evidence for galectin-3 as a key driver in fibrosis in many organs Directly addressing fibrosis not targeted by current experimental therapies 34

Summary 35

Pipeline and clinical development timeline GB0139 Galactic-1 IPF Phase 2b GB1211 Hepatic Impairment / Cirrhosis Phase 1b/2a GB2064 Myelofibrosis Phase 2a GB1211 NSCLC Phase 2a 2022 Enrollment complete Ph 2b top-line data Ph 2a top-line data Ph 2a top-line data Ph 2a top-line data 2023 Interim data Interim data 36

Galecto investment opportunity – well-funded with multiple shots on goal in oncology and fibrosis Galecto (NASDAQ: GLTO) is a clinical stage biotech developing small-molecule drugs targeting two essential proteins: Galectin-3: activator and recruiter of many cell types involved in fibrosis and cancer growth in the tumor micro-environment LOXL2: a key enzyme involved in the formation of the extracellular matrix in fibrosis and linked to worse prognosis in several cancers Galecto expects to have readouts from four Phase 2 programs by mid-2023 and is funded into 2H 2024 GB0139 – Phase 2b study in IPF (ongoing with top-line data expected in mid-2023) GB2064 – Phase 2a study in myelofibrosis (initiated in Q3 2021 and top-line data expected in 2H 2022) GB1211 – Phase 1b/2a study in liver cirrhosis (initiated in Q3 2021 and top-line data expected in 2H 2022) GB1211 – Phase 2a study in NSCLC in combination with checkpoint inhibitor (planned initiation in 1H 2022 and top-line data expected in mid-2023) 37
